Finally, add together the total mass of each element to get the molar mass of CO2: 12.0107 g/mol + 31.9988 g/mol = 44.0095 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in CO2, divide each total from step 3 by the total molar mass found in step 4: Mole Fraction. Percent.
Solved How many grams of carbon dioxide (CO2) are produced | Chegg.com
More information from the unit converter. How many moles Carbon Dioxide in 1 grams? The answer is 0.022722366761722. We assume you are converting between moles Carbon Dioxide and gram.You can view more details on each measurement unit: molecular weight of Carbon Dioxide or grams The molecular formula for Carbon Dioxide is CO2.The SI base unit for amount of substance is the mole. 1 mole is

Source Image: bartleby.com
Download Image
Sep 27, 2023The ratio of CO2 by mass can be found by dividing the mass of carbon (12 grams) by the molar mass of CO2 (44 grams/mol), which results in approximately 0.2727 or 27.27%. What is a lb of CO2? A lb of CO2 is a pound (unit of weight) of carbon dioxide gas.
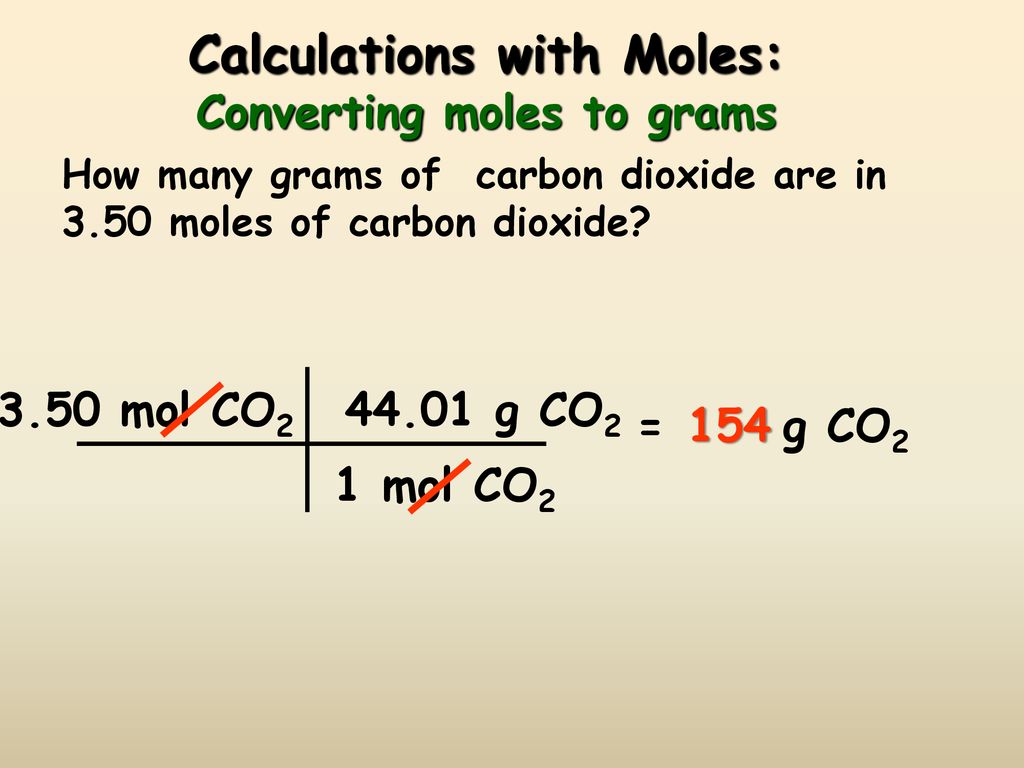
Source Image: slideplayer.com
Download Image
How many grams of carbon are present in 264g of CO2? – Quora How many grams of carbon are in 25g if CO2 – 16244902. jackm1516 jackm1516 30.03.2020 Chemistry Secondary School answered How many grams of carbon are in 25g if CO2 See answer Advertisement

Source Image: slideplayer.com
Download Image
How Many Grams Of Carbon Are In 25g Of Co2
How many grams of carbon are in 25g if CO2 – 16244902. jackm1516 jackm1516 30.03.2020 Chemistry Secondary School answered How many grams of carbon are in 25g if CO2 See answer Advertisement Apr 28, 2022Since there are 3 atoms in CO2 and 2 of them are oxygen, we will divide by 3 (1.14×10 23) and multiply by 2 (2.28 x10 23) So the number of oxygen atoms in 25.0 grams of CO2 is 2.28×10 23 or 228
CHEMICAL FORMULAE & REACTION QUANTITIES – ppt download
A compound CO2 of mass 25 grams. To Find : Amount of car on present in 25 grams of Carbon dioxide. Solution : •CO2 contains Carbon and oxygen. •Molecular weight of carbon is 12 grams. •Molecular weight of oxygen is 16 grams. •So, weight of carbon dioxide = 12 + (16×2) Weight of carbon dioxide=44 gra. Solved How many grams of carbon dioxide can be produced from | Chegg.com
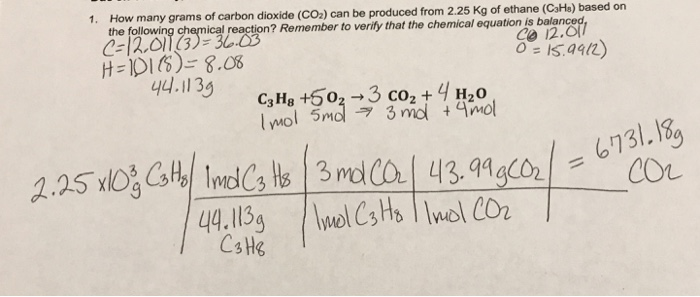
Source Image: chegg.com
Download Image
SOLVED: How many CO2 molecules are in 4.25 g of CO2? A compound CO2 of mass 25 grams. To Find : Amount of car on present in 25 grams of Carbon dioxide. Solution : •CO2 contains Carbon and oxygen. •Molecular weight of carbon is 12 grams. •Molecular weight of oxygen is 16 grams. •So, weight of carbon dioxide = 12 + (16×2) Weight of carbon dioxide=44 gra.
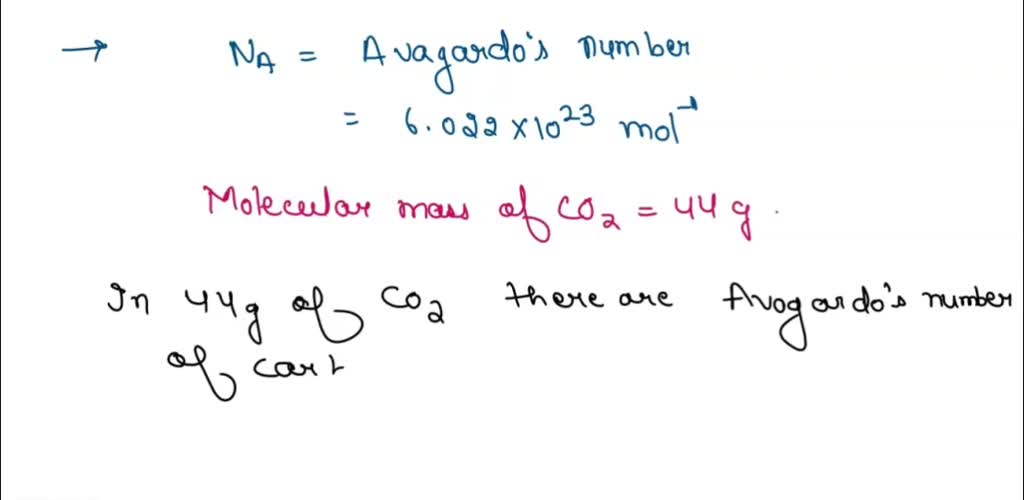
Source Image: numerade.com
Download Image
Solved How many grams of carbon dioxide (CO2) are produced | Chegg.com Finally, add together the total mass of each element to get the molar mass of CO2: 12.0107 g/mol + 31.9988 g/mol = 44.0095 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in CO2, divide each total from step 3 by the total molar mass found in step 4: Mole Fraction. Percent.
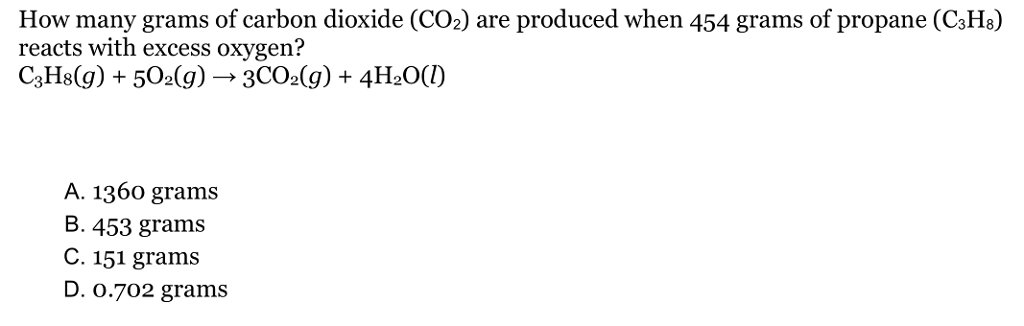
Source Image: chegg.com
Download Image
How many grams of carbon are present in 264g of CO2? – Quora Sep 27, 2023The ratio of CO2 by mass can be found by dividing the mass of carbon (12 grams) by the molar mass of CO2 (44 grams/mol), which results in approximately 0.2727 or 27.27%. What is a lb of CO2? A lb of CO2 is a pound (unit of weight) of carbon dioxide gas.
Source Image: quora.com
Download Image
How many molecules of carbon dioxide are present in 1g? – Quora Convert moles to grams and grams to moles. Substance formula. grams to moles. moles to grams. Mass of the substance, grams. Quantity of the substance, moles. Calculation precision. Digits after the decimal point: 3. Mass of the substance, grams.
Source Image: quora.com
Download Image
Dextrose 50% Injection: Package Insert – Drugs.com How many grams of carbon are in 25g if CO2 – 16244902. jackm1516 jackm1516 30.03.2020 Chemistry Secondary School answered How many grams of carbon are in 25g if CO2 See answer Advertisement

Source Image: drugs.com
Download Image
NFA 201 : Essentials of Nutrition – Eastern Kentucky University Apr 28, 2022Since there are 3 atoms in CO2 and 2 of them are oxygen, we will divide by 3 (1.14×10 23) and multiply by 2 (2.28 x10 23) So the number of oxygen atoms in 25.0 grams of CO2 is 2.28×10 23 or 228

Source Image: coursehero.com
Download Image
SOLVED: How many CO2 molecules are in 4.25 g of CO2?
NFA 201 : Essentials of Nutrition – Eastern Kentucky University More information from the unit converter. How many moles Carbon Dioxide in 1 grams? The answer is 0.022722366761722. We assume you are converting between moles Carbon Dioxide and gram.You can view more details on each measurement unit: molecular weight of Carbon Dioxide or grams The molecular formula for Carbon Dioxide is CO2.The SI base unit for amount of substance is the mole. 1 mole is
How many grams of carbon are present in 264g of CO2? – Quora Dextrose 50% Injection: Package Insert – Drugs.com Convert moles to grams and grams to moles. Substance formula. grams to moles. moles to grams. Mass of the substance, grams. Quantity of the substance, moles. Calculation precision. Digits after the decimal point: 3. Mass of the substance, grams.