Jun 23, 2023Hence, Total valence electrons in IF2– ion = valence electrons given by 1 iodine atom + valence electrons given by 2 fluorine atoms + 1 more electron is added due to 1 negative charge = 7 + 7 (2) + 1 = 22. Step 2: Select the central atom
IF2- Lewis Structure | How to Draw the Lewis Structure for IF2- | IF2- Lewis Structure | How to Draw the Lewis Structure for IF2- IF2- comprises one Iodine and two Fluorine
1. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is. Three fluorines are bonded to a central bromine. Each fluorine has three lone pairs, Bromine has two lone pairs. Once again, we have a compound that is an exception to the octet rule. 2.

Source Image: m.youtube.com
Download Image
In the IF 2- Lewis structure Iodine (I) is the least electronegative atom and goes in the center of the Lewis structure. The IF 2- Lewis structure you’ll need to put more than eight valence electrons on the Iodine atome. In the Lewis structure for IF 2- there are a total of 22 valence electrons. Watch on See the Big List of Lewis Structures
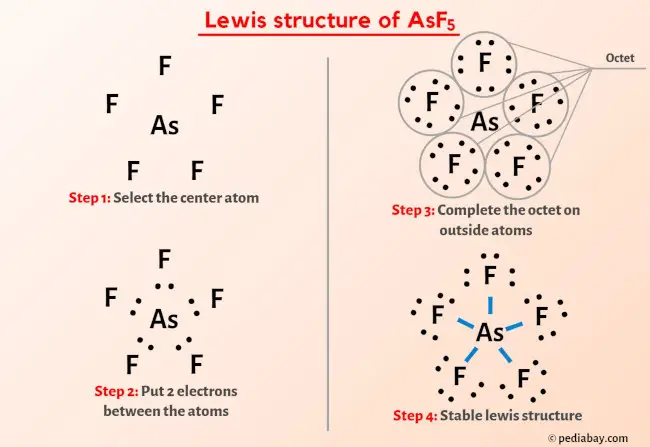
Source Image: pediabay.com
Download Image
Hybridization and Hybrid Orbitals | ChemTalk May 23, 2023So, let’s calculate this first. Calculation of valence electrons in IF2– ion For Iodine: Iodine is a group 17 element on the periodic table. [1] Hence, the valence electrons present in iodine is 7 (see below image). For Fluorine: Fluorine is a group 17 element on the periodic table. [2]
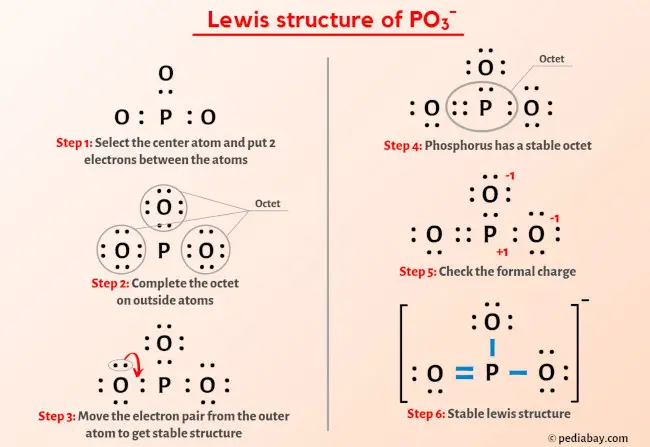
Source Image: pediabay.com
Download Image
What Is The Electron-Pair Geometry For I In If2-
May 23, 2023So, let’s calculate this first. Calculation of valence electrons in IF2– ion For Iodine: Iodine is a group 17 element on the periodic table. [1] Hence, the valence electrons present in iodine is 7 (see below image). For Fluorine: Fluorine is a group 17 element on the periodic table. [2] Figure 8.6.1 8.6. 1 shows the various molecular geometries for the five VESPR electronic geometries with 2 to 6 electron domains. When there are no lone pairs the molecular geometry is the electron (VESPR) geometry. When there are lone pairs, you need to look at the structure and recognize the names and bond angles.
PO3- Lewis Structure in 6 Steps (With Images)
May 9, 2022The formal charge on any atom can be calculated by the formula given below: Formal Charge (f) =V-B/2-N Where, V= No of valance electrons, B= No of bonding electrons, N= No of nonbonding electrons. Hence formal charge on I atom in IF2-=7-4/2-6= -1 Formal charge on each F atom in IF2– =7 -2/2-6= 0 OF2 Electron Geometry (Oxygen difluoride) – YouTube
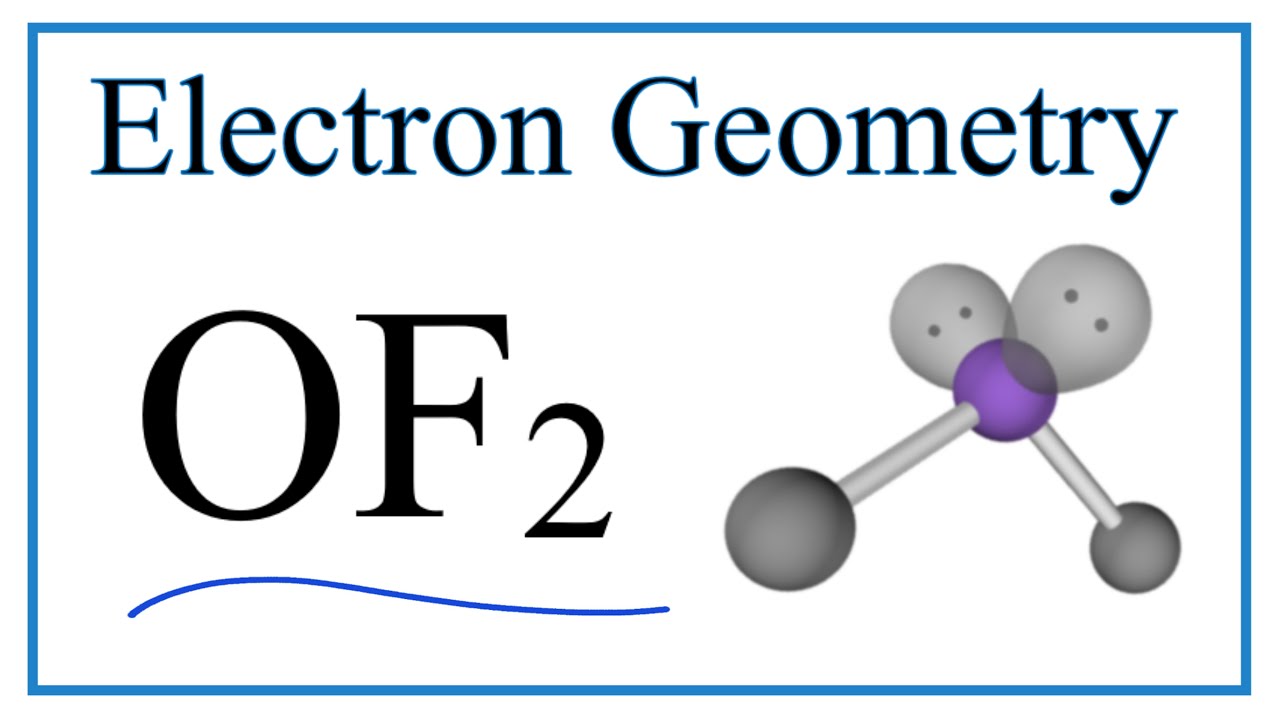
Source Image: youtube.com
Download Image
IF2- Lewis Structure in 6 Steps (With Images) May 9, 2022The formal charge on any atom can be calculated by the formula given below: Formal Charge (f) =V-B/2-N Where, V= No of valance electrons, B= No of bonding electrons, N= No of nonbonding electrons. Hence formal charge on I atom in IF2-=7-4/2-6= -1 Formal charge on each F atom in IF2– =7 -2/2-6= 0
Source Image: pediabay.com
Download Image
IF2- Lewis Structure | How to Draw the Lewis Structure for IF2- | IF2- Lewis Structure | How to Draw the Lewis Structure for IF2- IF2- comprises one Iodine and two Fluorine Jun 23, 2023Hence, Total valence electrons in IF2– ion = valence electrons given by 1 iodine atom + valence electrons given by 2 fluorine atoms + 1 more electron is added due to 1 negative charge = 7 + 7 (2) + 1 = 22. Step 2: Select the central atom

Source Image: facebook.com
Download Image
Hybridization and Hybrid Orbitals | ChemTalk In the IF 2- Lewis structure Iodine (I) is the least electronegative atom and goes in the center of the Lewis structure. The IF 2- Lewis structure you’ll need to put more than eight valence electrons on the Iodine atome. In the Lewis structure for IF 2- there are a total of 22 valence electrons. Watch on See the Big List of Lewis Structures

Source Image: chemistrytalk.org
Download Image
Class Notes Chemistry Hybridization | PDF Oct 20, 2023External links Steps Here’s how you can easily draw the IF 2- Lewis structure step by step: #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms #3 If needed, mention formal charges on the atoms Now, let’s take a closer look at each step mentioned above. #1 Draw a rough skeleton structure

Source Image: scribd.com
Download Image
What is the Lewis structure for IF2-? – YouTube May 23, 2023So, let’s calculate this first. Calculation of valence electrons in IF2– ion For Iodine: Iodine is a group 17 element on the periodic table. [1] Hence, the valence electrons present in iodine is 7 (see below image). For Fluorine: Fluorine is a group 17 element on the periodic table. [2]

Source Image: youtube.com
Download Image
7.92 | Identify the electron pair geometry and the molecular structure of each of the following – YouTube Figure 8.6.1 8.6. 1 shows the various molecular geometries for the five VESPR electronic geometries with 2 to 6 electron domains. When there are no lone pairs the molecular geometry is the electron (VESPR) geometry. When there are lone pairs, you need to look at the structure and recognize the names and bond angles.

Source Image: m.youtube.com
Download Image
IF2- Lewis Structure in 6 Steps (With Images)
7.92 | Identify the electron pair geometry and the molecular structure of each of the following – YouTube 1. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is. Three fluorines are bonded to a central bromine. Each fluorine has three lone pairs, Bromine has two lone pairs. Once again, we have a compound that is an exception to the octet rule. 2.
Hybridization and Hybrid Orbitals | ChemTalk What is the Lewis structure for IF2-? – YouTube Oct 20, 2023External links Steps Here’s how you can easily draw the IF 2- Lewis structure step by step: #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms #3 If needed, mention formal charges on the atoms Now, let’s take a closer look at each step mentioned above. #1 Draw a rough skeleton structure